Pfizer Inc. is recalling several lots of a blood pressure drug due to elevated levels of a potential cancer-causing impurity.
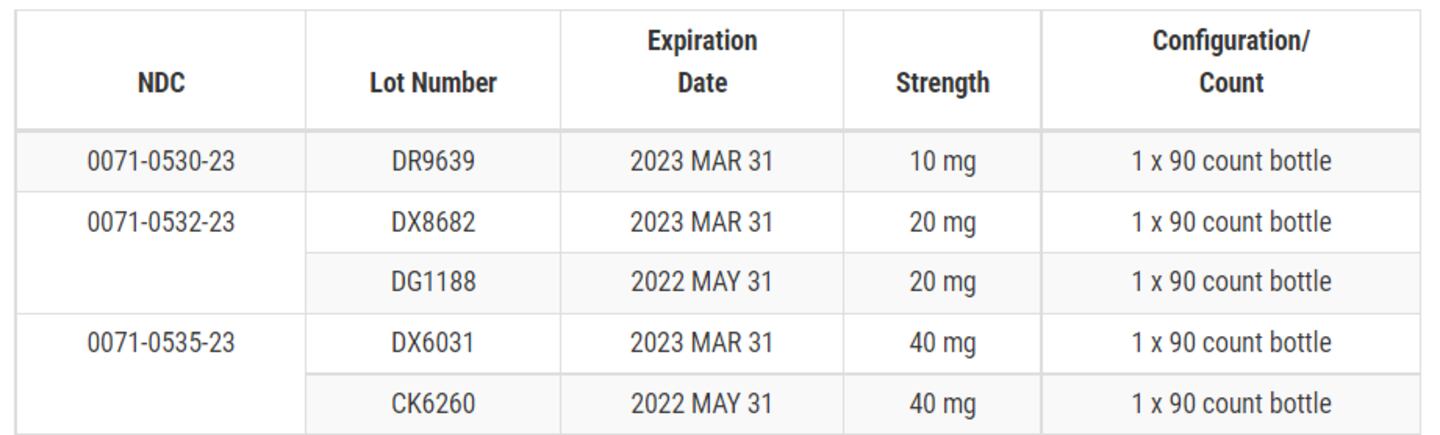
According to Friday’s announcement posted on the U.S. Food & Drug Administration website, Pfizer is voluntarily recalling five lots of Accupril tablets. Recent testing found the presence of a nitrosamine, N-nitroso-quinapril, above the Acceptable Daily Intake level.
According to the FDA, nitrosamines are common impurities, but long-term exposure at high levels may increase the risk of cancer. Pfizer says there have been no adverse reports received in relation to the recall and that there is no immediate risk to patients. Pfizer encourages patients to discuss options with their doctor.
The recalled product lots were distributed nationwide to wholesalers and distributors in the United States and Puerto Rico from December 2019 to April 2022, according to the FDA notice. The impacted products include:
Accupril® (Quinapril HCl Tablets), 10 mg
Accupril® (Quinapril HCl Tablets), 20 mg
Accupril® (Quinapril HCl Tablets), 40 mg
Patients in possession of recalled products seeking information on how to return products and obtain reimbursements should contact Sedgwick at 888-345-0481.
©2022 Cox Media Group